1Department of Applied Geology, Federal University of Technology, Nigeria
2Department of Chemistry, Ekiti State University, Nigeria
3Department of Earth Sciences, Ajayi Crowther University, Nigeria
*Corresponding author
Ayodele OS, Department of Applied Geology, Federal University of Technology, P.M.B 704, Akure, Nigeria, Tel: +2347060566760, E-mail: [email protected]
Int J Earth Sci Geophys, IJESG-3-010, (Volume 3, Issue 1), Research Article
Received: August 16, 2016
Accepted: June 15, 2017
Published: June 17, 2017
Citation: Ayodele OS, Awokunmi EE, Oshin OO (2017) Appraisal of Heavy Metals Pollution in the Stream Sediments from Okemesi-Ijero Area, Southwestern Nigeria: Insight from Geochemical Fractionations and Multivariate Analysis Techniques. Int J Earth Sci Geophys 3:010
Copyright: © 2017 Ayodele OS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The distribution and accumulation of heavy metals in the stream sediments of Okemesi-Ijero area, southwestern Nigeria was examined. Stream sediment samples from tensites were characterized for metals content such as Cadmium (Cd), Lead (Pb), Copper (Cu), Nickel (Ni), Iron (Fe), Manganese (Mn) and Zinc (Zn). The extractable heavy and macro metals such as, Cd, Pb, Cu, Zn, Ni, Fe and Mn in the stream sediment samples were analysed using Atomic Absorption Spectrometry (AAS). Apart from total concentration, the general distribution of these metals into five physico-chemical phases such as aqueous, exchangeable, bound to inorganic, bound to organic matter, and residual was explored using modified sequential chemical extraction scheme. The results showed higher vulnerability and bioavailability of Ni with non-lithogenous source. This was due to its higher percentage in the most mobile fraction. A significant amount of Cd, Cu, Mn and Fe in lithogenous fraction could be attributed to formation of stable complexes with Fe and Mn oxides. Considerable amount of Ni, Pb and Fe existed in the organic fraction due to their preference to the organic matters which often can form complex with humic substances. Principal Component Analysis (PCA) result reveals high concentration of Cd and Pb in study sites such as Odo-Owa-2, Ijero/Ipoti, Odo-Owa-5, Erigbe, Oke-Asa-1 and Arapate) which could be contributed by anthropogenic sources. This result pattern is consistent with clustering groups, which could provide more information about contributing sources. This study therefore suggests heavy metal contamination could be linked to the socio-economic activities.
Keywords
Assessment, Heavy metals, Stream sediment, Geochemical fractionations and Multivariate analysis
Introduction
Rapid population growth and urbanization in southwestern Nigeria is envisage to have led to the pollution of aquatic environment, due to largely uncontrolled contaminants discharge into the rivers ecosystem. In a river ecosystem, sediment is a main concern as related to issues such as water quality, aquatic habitat and reservoir water-storage capacity [1]. Sediment is an important source of heavy metal pollutants as they have a long residence time [2]. The pollution of sediments is closely linked to contamination of water by domestic sewage, untreated municipal wastewaters, industrial effluents and heavy traffic [3]. Sediment serves as a sink where contaminants can be stored and also act as a sink of the pollutants to the water body and inhabitants of the ecosystem (i.e. aquatic organism). Therefore, its transport and stockpile heavy metals and consequently reveals the quality of the water system [4]. The concentrations of heavy metals in sediments depend not only on anthropogenic/non-lithogenic and lithogenic sources. Nonetheless, it's also depends on the grain size, organic matter, mineralogy and depositional environment of the sediments (Kayastha, 2015 and reference there in) [3]. Heavy metals pollution is a major environmental challenge throughout the world. This is due to its destructive and harmful effects on living organisms when their concentrations exceed a permissible limits [5,6].
Heavy metals previously fixed on sediments could be release to the water body due to changes in environmental conditions. For example pH, redox potential and chelation in the aquatic ecosystem [7]. The mobility and bio-accessibility of heavy metals depends on their chemical forms or type of the binding. This practice is very appropriate for site assessment and risk analysis [8]. Thus, the physico-chemical forms of the metals in the sediment are of great importance in evaluating their mobility potential [9]. Bioavailability and mobility of heavy metals in aquatic environment is not appropriately describe by total metal concentration [10,11]. The determination of the total concentration of the metals gives no indications on their various forms [12,13]. Therefore, sequential chemical extraction approach have been considered, developed and applied to the aquatic sediments. Sequential chemical extractions, although rigorous, furnishes comprehensive information about the source, mode of occurrence, biological and physico-chemical availability, mobility and transport of trace metals. Chemical extraction is very useful for evaluating the degree of association of the metals and also determines the extent of remobilisation in the environment [3]. Furthermore, it could be used for distinguishing between those metals from lithogenic and non-lithogenous (i.e. anthropogenic sources).
The purpose of this study is to appraise distribution and accumulation of heavy metals in different physico-chemical phases in the sediments of the rivers around Okemesi-Ijero areas, southwestern Nigeria. Sediment samples were obtained from ten locations and analysed for metal content which includes Cd, Pb, Cu, Zn, Ni, Fe and Mn.
Accessibility and Drainage System
The study area lies within Latitudes 70 45'N and 80 00'N and Longitudes 40 52'E and 50 08'E. The study areas is part of the topographic map sheet No. 243 (Ilesha N.E. 1:50,000) and sheet No. 244 (Ado N.W. 1:50,000). The areas cover parts of Ekiti and Osun States, southwestern Nigeria with a total surface area of 821.4 km2. Accessibility is through network of all seasonal roads and motorable tracks which links it with other part of the country. Similarly, villages and towns have major and minor roads and also footpaths which are inter-linked to one another. The streams spread out from a central point forming dendritic drainage pattern as a result of its branching, they develop where the cover channels follow the slope of the terrain. Dendritic system forms in V-shaped valleys as a result of the rock types whether porous on non-porous, while the drainage pattern where the topography is dominated by series of ridges is the trellis type which suggests that the drainage is structurally controlled. Major rivers in the study area includes River Osun which is found along Okemesi road, River Oyi which flows in a southerly direction. A waterfall was encountered around Oke-Ila called Ayikunnugba waterfalls and river Isa etc. Most of the rivers are actively flowing but some are stagnant and others have dried out channels attributed to direct evaporation during dry season. The nature of rivers is controlled by the gradient (slope), the prevailing climatic conditions, the structural features (such as joints, fractures, veins and foliations), geomorphology and physiology and the lithology [14].
Materials and Methods
The sampling density of one sample per 4 sq km2 for the collection of stream sediments samples (Figure 1). Samples were obtained at a depth of 20-25 cm; they were bagged and labeled to avoid mix up before transportation to the laboratory. The geographical locations of each sample collected and characteristic features of the stream sediments were noted. The samples were pulverize and milled into homogenous and fine materials.
Total acid digestion
Pulverised samples (0.1 g) are dissolved using 4 ml of an oxidizing mixture (HNO3: HCl = 3:1) and 6 ml HF was added. The solution in a Teflon recipient was put in a microwave oven (800 w, 4 min; 20 min. of ventilation). The samples were treated with 5.6 g HBO3 to avoid silica evaporation and diluted to 100 ml by distilled/de-ionized water. The solution were analysed for selected metals using Atomic Absorption Spectrometer (AAS, Perkin Elmer Model 306).
Modified single sequential extraction technique
A five-step modified chemical extraction procedure was applied to evaluate heavy metal fractions (chemical phases) in the river sediments. The method is designed/formulated to separate the heavy metals into the following five fractions: 5.2.1. Aqueous phase (Step I):
Aqueous phase (Step I)
45 ml of 1 M ammonium acetate adjusted to pH5 with acetic acid was added to 2.5 g of samples. The mixture was stirred for 24 hrs at a room temperature. The suspension was then centrifuged at 300 rpm for 20 min, filtered and diluted to 100 ml with distilled/deionised water. The solution was analyzed for considered metals using Atomic Absorption Spectroscopy (AAS).
Exchangeable phase (Step II)
22.5 ml of hydroxyl ammonium acetate was added to residue from previous step and followed by acetic acid (25%). After 24 hrs stirring at a room temperature, the solution was centrifuged and filtered through micro pore membrane. The solution was diluted to 100 ml by deionized water and analyzed for heavy metals using AAS.
Inorganic phase (Step III)
12.5 ml of HCl (0.1 M) was added to residue from exchangeable phase 2 and stirred for 24 hrs at room temperature. The solution was centrifuged, filtered through micro pore membrane. The solution was diluted to 100 ml with deionised water and analysed for heavy metals using AAS.
Organic phase (Step IV)
12.5 ml of NaOH (0.5 M) was added to residue from previous step and stirred for 24 hrs at a room temperature. For sediment samples with large organic content, this procedure was repeated until a clear solution was obtained. All the solutions separated from the solid were then dried using IR lamp at 60 °C and dissolved using 4 ml of HNO3 (65% ) and 2 ml HF (40%) in a microwave oven (250 w, 1 min; ow, 2 min; 250 w, 5 min; 400 w, 5 min; 600 w, 5 min). The solution was diluted to 25 ml and analyzed for heavy metals using AAS.
Residual (Step V)
12.5 ml of HNO3 (8 M) was added to residue from previous step and digested for 3 hrs at 80 °C. The solution was diluted to 25 ml and analyzed for heavy metals using AAS. The residual solid from the step V was finally digested as described in section 3.6.2. The analysis was done at Atomic Absorption Spectrophotometry (AAS) Laboratory, Centre for Research and Development, Obafemi Awolowo University (O.A.U.) Ile-Ife, Nigeria.
Multivariate statistical analysis
Principal Component Analysis (PCA) is as an unverified pattern recognition method using Varimax rotation and Kaiser normalization. This is extensively used for data reduction and extraction of small number of principal components. This is used for investigating the probable similar distribution behaviour of metals and analyzing relationships among the numerous variables [15]. PCA is used to evaluate interactions among the variables and identify heavy metals pollution sources. In this study, it is carried out using the SPSS V17.0 for Windows. Cluster analysis combines all variables together and could be used to trace similarities and differences of them. This could give more clues to further corroborate the results of the PCA analysis. Dendogram clusters the elements using Euclidian distance and Ward procedure [16]. This is illustrated as horizontal dendogram (Figure 2).
Results and Discussion
Total metal content
Metal contents in the stream sediments from Okemesi-Ijero areas are shown in Table 1. It is evidently shown from the data that the concentrations of the seven metals varied as follows: Cd, 1.8-2.8 m kg-1; Cu, 3.0-4.8 mg kg-1, Pb, 1.6-3.0 mg kg-1, Zn, 2.1-3.8 mg kg-1, Ni, 12.3-18.9 mg kg-1 and Fe, 29.7-55.1 mg kg-1. The average content of Mn, Ni and Pb are lower than those documented in a study done by Akintola, et al. (2014) [17]. Conversely, the mean Zn concentration obtained is higher than those reported from stream sediments contaminated by mining activities in Ibodi, southwestern, Nigeria [17]. The average contents of all considered metals decrease in the order of Fe > Ni > Mn > Cu > Zn > Cd > Pb. In order to appraise the likely environmental concerns of the selected metals, results shown in Table 1 were compared to US NOAA's sediment quality guidelines (Table 2) [18,19]. In this study, the Effects Range-Low (ERL) and Effects Range-Median (ERM) contents was used. The ERL signifies chemical contents below which adverse biological effects were rarely seen but ERM characterizes contents above which effects were more usually observed. Mostly, the adverse effects take place in < 10% of studies in which concentrations were below the respective ERL values. On the other hand, it was observed in > 75% of studies in which concentrations exceeded EMR values [20]. ELR and EMR values for the metals are shown in Table 2. All the investigated metals have lower concentration when compared with ERM values. Likewise, all metals with the exception of Cd showed lower concentration when compared with ERL. In the case of Cd, all the studied sites showed higher concentration than the ERL values. Turki (2007) [19] indicated that the toxicity is a function also of the degree to which data exceed ERM values. Therefore, some environmental or toxicological effects of Cd studied sites, cannot be expected.
Table 1. Contents of macro and trace elements in the studied samples.
Chemical fractionation results
Figure 3a and Figure 3b depicts the distribution of the studied metals in the five extractable fractions in percent bar graphs. The partitioning reveals that the percentages of metals associated with the non-lithogenous fractions (aqueous + exchangeable + adsorbed on inorganics + associated organics) (Table 3a and Table 3b) were notably greater for Cd, Pb, Ni, Mn and Fein all studied sites than those of the lithogenous fraction. This indicated that these metals were predominantly derived from anthropogenic inputs somewhat than the geochemical sources. On the other hand, Cu is considerably greater in lithogenous fraction that non-lithogenous fraction indicating geochemical sources. Therefore, the results show that such metals are possibly available for exchange and/or release into the coastal environment. The selected metals in the non-lithogenous fractions increased in the order of Cu > Pb > Cd > Zn > Mn > Ni > Fe (Table 4).
Cadmium
The sediments lithogenous fraction is dominated by Cd, which accounts for over 31.9% of the total Cd concentration. Among the non-lithogenous fractions, the adsorbed on inorganic phases was much significant than other fractions, which account for about 30% of the total Cd. The second in the order is aqueous phase which accounted for 15.3% of the total Cd concentration. The absorption at the organics phase is higher than the exchangeable phase (Figure 3a and Table 3a). The insignificant amount of Cd at the exchangeable phase indicates lesser amount of bioavailable cadmium. From the abovementioned fact, it can be indicated that the Cd is bound to the inorganic phase (i.e. carbonate phases). This suggestion is in accordance with the available literature data [21].
Copper
Cu dominates the lithogenous fraction of the sediments, accounting for 50.9% of the total Cu concentration. This indicates that a considerable amount of Cu is expectedly bound to aluminosilicate minerals. This bounded Cu is somewhat unaffected towards the change of environment conditions. This is followed by exchangeable phase among the non-lithogenous fraction, with average percentage of 14.9% of the total Cu concentration. Absorption at the bound to inorganic phases accounted for 13.9%, this is followed by bound to organic phases (12.1%) (Figure 3a and Table 3a). The least percentage of Cu is found in the bound to aqueous phase (i.e. 8.2%) of the sediments. In spite of high stability constants of the organic Cu compounds [22,21], the organic matters bound Cu seemed to be less pronounced which could be attributed to low content of the organic matters. Therefore, low organic matters content in sediment suggest that the residual fraction could significantly binds copper.
Table 3a. The mean percentage proportion of metals (%) in the non-lithogenous and lithogenous fractions.
Lead
Substantial contents of Pb were present in the aqueous phase (i.e. 31.3%) of non-lithogenous fractions. This is followed by the lithogenous fraction, which accounts for about 20.8% of the total Pb content. The % of Pb associated with other non-lithogenous fractions in all samples was in the order: exchangeable (20.2%) > organics (17.5%) > inorganic (10.1%) (Figure 3a and Table 3a). Pb is tightly bound by sulfide mineral under reducing conditions through precipitation and complexion with insoluble organic matter. On the other hand, it is immobilised by precipitated iron oxide under well-oxidized conditions [23]. Inorganic Pb forms compounds such as sulfide, carbonate, and sulfate minerals. These are universally abundant in sediment nevertheless have low solubilities in natural water. Relative abundance of Pb in the easily mobilised (i.e. exchangeable) fraction agreed with the available data in the literature [21].
Zinc
Substantial percentage of Zn was extracted from aqueous phase (i.e. 30.82%) of the sediments. The amounts of Zn associated with other non-lithogenous fractions in all samples were in the order: exchangeable (22.42%) > inorganic (19.65%) > organic (16.95%) (Figure 3a and Table 3a). Although, the percentage of Zn associated with lithogenous fraction accounts for 10.24% of Zn concentration. The inorganic phase are metals associated with sulfide, sulfate and carbonates; the CaCO3 which is strong absorbent and form complexes with Zn as double salts (i.e. CaCO3.ZnCO3). Zinc co-precipitate with the carbonates which could become an important chemical form; especially when the hydrous iron oxide and the organic matter are less abundant. Zinc is relatively more mobile when compared with Pb and is freely adsorbed by clay minerals, and carbonates or hydrous oxides [24].
Nickel
In this study, large amounts of Ni were concentrated in the exchangeable phase (35.49%) of the samples. The amount of Ni in other non-lithogenous fraction in all samples was in the order: organic phase (29.36%) > aqueous phase (12.07%) > inorganic phase (10.39%) (Figure 3b and Table 3b). The lithogenous fraction in the sediments accounts for over 12.96% of total Ni concentration. This is consistent with available data in literature [25]. Substantial amount of Ni in the exchangeable fraction could be due to anthropogenic inputs (i.e. combustion of fossil fuels). Nickel contents could be added by bedrocks as well as anthropogenic sources [26,25]. This is in accordance with the previous study [21]. Metals bound to exchangeable fraction can be release to body of water in the suspended or dissolved form. Thus, presents an eventual danger for an aquatic ecosystem [27].
Table 3b. The mean percentage proportion of metals (%) in the non-lithogenous and lithogenous fractions (Contd.)
Manganese and Iron
Manganese is mostly concentrated in the lithogenous fraction of the sediments, which accounts for over 50% of total Mn concentration. The amounts of Mn in the non-lithogenous fraction of all samples was in the order: inorganic phase (20.59%) > exchangeable phase (14.71%) > organic phase (8.82%) > aqueous phase (5.88%) (Figure 3b and Table 3b). The sediments have more Mn in the not easily mobilized fraction. In the case of Fe, is typically concentrated in the lithogenous fraction. This accounts for over 37.64% of total Mn concentration. The average contents of Fe in the non-lithogenous fraction of all samples was in the order: aqueous phase (24.54%) > organic phase (24.22%) > exchangeable phase (8.77%) > inorganic phase (4.83%). The recoverable amount of Fe in the exchangeable fraction is very low but Mn is relatively high in the exchangeable fraction. This is in accordance with available data in the literature [27].
Multivariate analysis results
The obtained geochemical extraction data for the ten analysed samples (Table 4) were submitted for multivariate analysis using SPSS 17 version. The factor analysis would outline the importance of Fe and Mn as indicators for forecasting the necessary behaviour of the selected metals. The factor showed exchangeable metals together with the corresponding variance values. This represents the proportion of the samples which could be divided only on the basis of selected metals. The results of the factor analysis after varimax rotation were proved to be more appropriate for application. In this study, the loadings which are higher than 0.4 are used for describing a factor.
Table 4. Averages and medians of metal contents in five geochemical fractions (mg Kg-1).
Metals bound to aqueous phase
Macro and micro metals extracted in the aqueous phase (Figure 2) are divided into two clusters. First cluster is Ni, which is distant from other cluster, and very near to the point of Fe which suggests that Ni could be bound to amorphous iron oxyhydroxide. The second cluster is Zn, Mn, Cu, Pb and Cd which are connected in the dendogram. This indicates their close connection and similar nature. The principal component analysis of aqueous fractions could not be computed because of zero variance (Table 5).
Metals bound to exchangeable phase
Factor loading values for the exchangeable metals in components I, II and III are shown in Table 5. Component I (Table 5) represents 32.94% of the total data variations and have high positive loadings for Ni and Cu. Nevertheless, they show different behaviour within the same groups of cluster suggesting different sources. Component II (Table 5) contributes 31.84% to the total data variation and have high positive loadings for Zn and Fe. Iron is near to zinc which suggests a similar behaviour and source for both metals (Figure 2). Component III (Table 5) accounts for 19.42% of the total data variation and have strong loadings for Pb and Fe but showed similar behaviour within the same groups of cluster (Figure 2). Metals bound to exchangeable are separated into two clusters. First cluster shows Ni which is very far from other cluster and very close to Fe. This indicates a similar nature for metals in most mobile fraction. Second cluster comprises of Zn, Mn, Cu, Pb and Cd indicating their close correlation and comparable nature (Figure 2). This fraction contains elements that are mobile and consequently pose more vulnerability to the environment.
Metals bound to inorganic phase
Metals bound to inorganic phase are shown in Table 5. Component I (Table 5) accounts for 25.56% of the total data variation and strong loadings for Cu and Pb. Their similar behaviour is indicated inside the same groups of cluster (Figure 2). Component II (Table 5) represents 25.55% of the total data variation and described by strong loadings for Ni and Zn. Their different behaviour depicted in the same groups of cluster (Figure 2). Component III (Table 5) contributes 22.11 to the total variance and has strong loadings only for Fe suggesting a different nature. The same conclusion can be obviously seen from the dendogram (Figure 2). Selected metals obtained in the inorganic fractions (Table 5 and Figure 2) are separated into two clusters. First cluster is Ni, is far away from other cluster, and very adjacent to the point of Fe. This indicated that Ni could be bound to amorphous iron oxyhydroxide. The second cluster consists of Pb, Cd, Cu and Zn which is adjacent to manganese which can be carbonate co-precipitated with Mn-oxyhydroxides (Figure 2).
Table 5. Varimax rotated factor loadings matrix and communalities obtained from principal component analysis for five geochemical fractions from stream sediments.
Metals bound to organic phase
The obtained factor loadings value of metals bound to organic phase are shown in Table 5. Component I (Table 5) accounts for 32.05% of the total data variation and described by positive loadings of Fe and Mn. Their similar behaviour in the same groups of cluster is shown in Figure 2. Component II (Table 5) contributes 29.30% to the total variance and have positive loadings of Zn and Cd, both have similar behaviour inside the same groups of cluster (Figure 2). Component III (Table 5) represents 22.08% of the total data variation and have positive loadings for Zn and Cu. They both have similar behaviour in the same groups of cluster (Figure 2). Metals extracted from organic phase are separated into two clusters. One is Fe, which is far from other cluster, and very close to Mn. Second cluster includes Pb, Cu, Cd and Zn which could be in nature with insoluble organic matter (Figure 2).
Metals bound to residual phase
The obtained factor In the residual phase, the dissolution of crystalline Fe-oxide is relatively complemented by alumino-silicates destruction [28]. Table 1 indicates iron as the main constituent (Table 1). The obtained loading values for metals bound to residual phase are shown in Table 5. Component I (Table 5) contributes 34.73% to the total variance of the data. It has strong association only for Fe which suggests a different nature of Fe. This conclusion is supported by the behaviour of Fe within the same of clusters in dendogram (Figure 2).
Component II (Table 5) represents 34.04% of the total data variation and consist strong loadings of Cu, Cd and Zn. Cadmium is adjacent to Cu which suggest comparable source. Zinc has similar behaviour inside the same groups of cluster (Figure 2). Based on the dendogram for the metals associated to the residual phase (i.e. detritus silicates, crystalline Fe oxide) as indicated in Figure 2. It is expected to conclude that all selected metals are associated with crystalline Fe-oxide and silicates.
Conclusions
Macro and micro metal geochemical phases and principal component analysis have been employed for evaluation of river sediments from Okemesi-Ijero area, southwestern, Nigeria. The results indicate high contents of Zn, Cd, Pb, and Ni. This could be ascribed to the anthropogenic inputs from indiscriminate discharge of contaminants and traffic pollution. The above average contents of the selected metals are in the order: Ni: Odo Owa 5 > Arapate > Lawrence; Cd: Lawrence 1 > Odo Owa 2 > Ijero/Ipoti > Odo Owa 5; Pb: Erigbe > Oke Asa 1 > Arapate > Odo Owa 5; Zn: Ijero/Ipoti > Odo Owa 2 > Erigbe > Ipoti 3. The distribution of Cu in the studied sites is controlled by geochemical background. The above average contents of the Cu in considered samples are in the order: Ipoti 3 > Arapate > Odo Owa 5 > Odo Owa 2 > Lawrence 1. Heavy metal pollution is of great concerns because of their toxicity poses serious dangers to human health and the aquatic ecosystem. The study thus recommended that, these rivers should be put under surveillance to prevent indiscriminate discharge of domestic sewage.
Acknowledgements
We appreciate the technical assistance of final year students who participated in the field work (2014/2015 session). The authors also thank the staff of Atomic Absorption Spectrophotometry Laboratory, CRD, Obafemi Awolowo University, Ile-Ife, Nigeria for metal analysis.
Figures
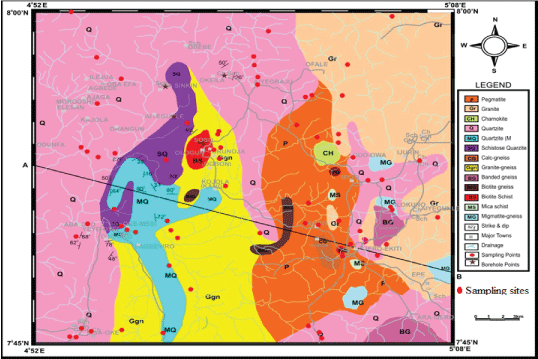
Figure 1: Stream sediment sampling points.
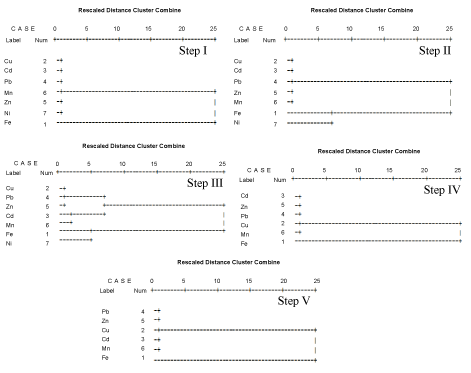
Figure 2: Dendrogram cluster analysis of five geochemical fractions from stream sediments.
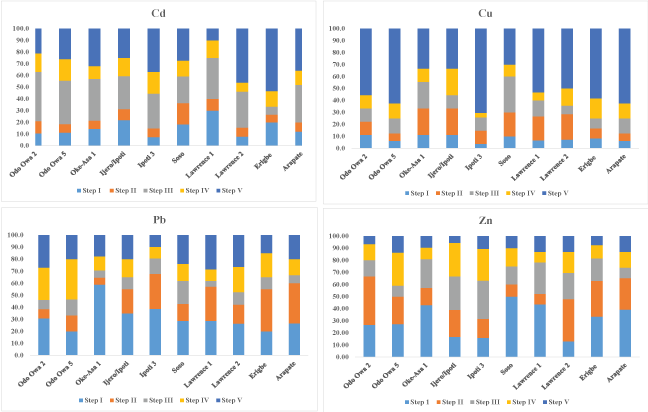
Figure 3a: Percentage distribution of Cd, Cu, Pb and Zn in the five geochemical phases in stream sediments.
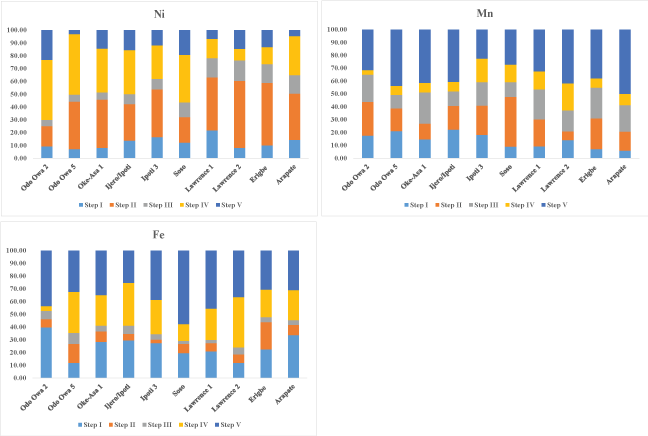
Figure 3b: (contd.). Percentage distribution of Ni, Mn and Fe in the five geochemical phases in stream sediments.
References
-
Juracek KE, Ziegler AC (2009) Estimation of sediment sources using selected chemical tracers in the Perry lake basin, Kansas, USA. Intern J Sediment Research 24: 108-125.
-
Forstner U, Wittman GTW (1983) Metal Pollution in Aquatic Environment. Springer Verlag Berlin, Heidelberg, New York.
-
Kayastha SP (2015) Heavy metals Fractionation in Bagmati River Sediments, Nepal. Journal of Hydrology and Meteorology 9: 119-128.
-
Chapman MP, Wang F (1999) Biological Implications of Sulfide in Sediment- a reviewing focusing on sediment toxicity. Environmental Toxicology 18: 2526-2532.
-
MacFarlane GR, Burchette MD (2000) Cellular distribution of Cu, Pb and Zn in the Grey Mangroove, Avicennia marina (Forsk.) Vierh. Aqua Bot 68: 45-59.
-
Pekey H (2006) The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Marine Pollution Bulletin 52: 1197-1208.
-
Sutherland RA, Tack FMG (2007) Sequential extraction of lead from grain size fractionated river sediments using the optimized BCR Procedure. Water Air Soil Pollut 184: 269-284.
-
Howard JL, Shu J (1996) Sequential extraction analysis of heavy metals using a chelating agent (NTA) to counteract resorption. Environ Pollut 91: 89-96.
-
Lopez-Gonzalez N, Borrego J, Morales JA, Carro B, Lozano-Soria O (2006) Metal fractionation in Oxic sediments of an estuary affected by acid mine drainage (south western Spain). Estuar Coast Shelf Sci 68: 297-304.
-
Singh M, Muller G, Singh IB (2003) Geogenic distribution and baseline concentration of heavy metals in sediments of the Ganges River, India. J Geochem Explor 80: 1-17.
-
Zakir HM, Shikazono N, Otomo K (2008) Geochemical distribution of trace metals and assessment of anthropogenic pollution in sediments of Old Nakagawa River, Tokyo, Japan. Am J Environ Sci 4: 654-665.
-
Tokalioglu S, Kartal S, Elçi L (2000) Determination of heavy metals and their speciation in lake sediments by flame atomic absorption spectrometry after a four-stage sequential extraction procedure. Anal Chimica Acta 413: 33-40.
-
Marengo E, Gennaro MC, Robotti E, Rossanigo P, Rinaudo, et al. (2006) Investigation of anthropic effects connected with metal ions concentration, organic matter and grain size in Bormida river sediments. Anal Chim Acta 560: 172-183.
-
Akanni CO (1992) Aspect of Climate in Ogun State in maps. In: Onakomuye SO, Oyesiku, Jegede, FJ Ife Journal of Science 4: 5-6.
-
Loska K, Wiechuła D (2003) Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 51: 723-733.
-
Laursen J, Milman N, Pind N, Pedersen H, Mulvad G (2014) The association between content of the elements S, Cl, K, Fe, Cu, Zn and Br in normal and cirrhotic liver tissue from Danes and Greenlandic Inuit examined by dual hierarchical clustering analysis. J Trace Elem Med Biol 28: 50-55.
-
Akintola AI, Ikhane PR, Bankole SI, Mosebolatan OA (2014) Preliminary Investigation of Stream Sediments Contaminations Caused by Mining Activities in Ibodi and Its Environs, S/W Nigeria Using Geological and Geochemical Assessment Approach. Environment and Natural Resources Research 4: 16-27.
-
Ramirez M, Serena M, Frache R, Correa J (2005) Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar Pollut Bull 50: 62-72.
-
Turki AJ (2007) Metal Speciation (Cd, Cu, Pb and Zn) in Sediments from Al Shabab Lagoon, Jeddah, Saudi Arabia. JKAU: Mar Sci 18: 191-210.
-
Long ER, Field LJ, McDoland DD (1997) Predicting toxicity in marine sediments with numerical sediment quality guidelines. Environ Toxicol Chem 17: 714-727.
-
Todorović ZB, Ranđelović LM, Marjanović JZ, Todorović VM, Cakić MD, et al. (2014) The assessment and distribution of heavy metals in surface sediments from the reservoir "Barje" (Serbia). Advanced Technology 3: 85-95.
-
Han BC, Jeing WL, Hung TS, Wen MY (1996) Relationship between copper speciation in sediments and bioaccumulation by matine bivalves of Taiwan. Environmental Pollution 91: 35-39.
-
Gambrell RP, Wiesepape JB, Patrick WH Jr, Duff MC (1991) The effects of pH, redox, and salinity on metal release from a contaminated sediment. Water, Air, and Soil Pollution 57: 359-367.
-
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J Environ Manage 90: 1106-1116.
-
Ma Xi, Zuoa H, Tiana M, Zhanga L, Menga J, et al. (2016) Assessment of heavy metals contamination in sediments from three adjacent regions of the Yellow River using metal chemical fractions and multivariate analysis techniques. Chemosphere 144: 264-272.
-
Adamo P, Dudka S, Wilson MJ, McHardy WJ (1996) Chemical and mineralogical forms of Cu and Ni in contaminated soils from the Sudbury mining and smelting region, Canada. Environ Pollut 91: 11-19.
-
Zakir HM, Shikazono N (2011) Environmental mobility and geochemical partitioning of Fe, Mn, Co, Ni and Mo in sediments of an urban river. Journal of Environmental Chemistry and Ecotoxicology 3: 116-126.
-
Polić P, Pfendt P (1996) Alluvial aquifer contamination: Carbonates and easily reducible oxyhydroxides as heavy metal substrates. Journal of the Serbian Chemical Society 61: 1001-1013.
