International Journal of Metallurgy and Metal Physics
Hydrogen Effect on Creation of Nanoporous Palladium by Palladium Hydride Wire Explosion
J Baker*, J Gahl, P Norgard, O Azizi, A El-Boher, G Hubler, J He, W Isaacson, and D Pease
Sidney Kimmel Institute for Nuclear Renaissance (SKINR), University of Missouri, USA
*Corresponding author
J Baker, Sidney Kimmel Institute for Nuclear Renaissance, University of Missouri, 349 Engineering Building West, Missouri 65211, USA, E-mail: [email protected]
Int J Metall Met Phys, IJMMP-1-002, (Volume 1, Issue 1), Letter
Received: August 28, 2015
Accepted: October 15, 2015
Published: October 17, 2015
Citation: Baker J, Gahl J, Norgard P, Azizi O, El-Boher A, et al. (2015) Hydrogen Effect on Creation of Nanoporous Palladium by Palladium Hydride Wire Explosion. Int J Metall Met Phys 1:002
Copyright: © 2015 Baker J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Nanoporous palladium was created using highly loaded palladium hydride wires subjected to a fast electrical pulse of energy. The delivered energy was insufficient to melt an unloaded palladium wire. Electrical explosion experiments of palladium hydride wires were performed on single samples at the loading ratios ranging from 0.5 up to 0.96, approaching the highest experimentally achieved loading ratio of 1. It was found that nanoporous palladium was created by pulsing of palladium hydride wires at loading ratios higher than the threshold of 0.6. Each additional increase in the hydrogen loading ratio caused an accompanying increase in the surface area. In contrast, when the hydrogen loading ratio was below 0.6 the wire remained intact and there was no nanoporosity produced. This novel method used a low energy, but high power, fast rise time pulsed power system which is effective in creating nanoporous palladium.
Keywords
Nanoporosity, Palladium hydride, Deuterium, Pulsed power
Introduction
Nanoporous materials have recently attracted considerable attention due to their critical importance in technological applications. One such material is nanoporous palladium which not only serves as a catalyst but is also useful in hydrogen storage and sensing [1-3]. For instance, a large surface to volume ratio of nanoporous palladium has been utilized in the electrocatalytic hydrogenolysis of chlorinated organic compounds [4]. A number of groups have shown that nanoporous palladium can be produced using a variety of dealloying processes [5,6]. The evolution of the porous structure during dealloying is dominated by both dissolution of the less noble component and surface diffusion of the more noble component. It is difficult to fully remove the less noble element in this process, leaving the residuals of less noble element in the nanoporous palladium.
Nanoporous palladium powders were also synthesized by chemical reduction of tetrachloro complexes by ascorbate in a concentrated aqueous surfactant [7]. This method can produce porous structures with pore sizes of 2 - 3 nm.
Recently, we discovered a novel, nonchemical, method to create nanoporous palladium through an electrical wire explosion [8]. Others have used electrical wire explosions to produce metal nanopowders, but not nanoporous metals [9]. In our experiment, with the intended application to high energy density physics, deuterium loaded wires were pulsed with a low energy pulse, a so called pre-pulse, with the intent to liberate the deuterium from the wire. Surprisingly, the loaded wires were converted into nanoporous palladium. On the contrary, no porosity was observed in the pulsing of palladium wires without loaded deuterium. In fact, the unloaded wires survived the pulse intact. It thus appears that the deuterium played a critical role in the formation of nanoporous palladium. In that experiment, the highly loaded palladium deuteride wires were chemically sealed using electrodeposited mercury during electrochemical loading. After reaching high deuterium loading, a monolayer of mercury was formed on the palladium surface using electrochemical deposition. This monolayer of mercury blocks active sites for the deuterium adsorption/reduction reaction, while promoting deuterium or hydrogen absorption by inhibiting the recombination reaction [10]. Although mercury proves to be good in keeping a high deuterium loading ratio, it is toxic and can also negatively impact the effectiveness of catalysts thereby reducing the yield of reactions. Furthermore, it was not clear how the deuterium or mercury affected porosity formation in the deuterium loaded wires.
Materials and Methods
In this letter, we report the creation of nanoporous palladium through electrical explosion of palladium hydrides wires without using mercury and study the effect of hydrogen loading ratios on the formation of nanoporosity in palladium. The experiments consist of two parts. First, we loaded palladium wires with hydrogen at varying concentrations through electrolysis. 99.9% pure palladium wires (Alfa Aesar) of approximately 3.75 cm in length and 50 μm in diameter were annealed in air for the annihilation of defects. A small amount of oxides may be produced on the surfaces of the wire, but they would be reduced in electrolysis and form roughed surface morphologies, which are believed to be beneficial to the hydrogen loading process. The annealing was achieved by ramping electric current through the wire from 0.5 A up to 0.75 A. The current was then ramped back down again in a similar fashion. The total time of annealing was 17 min. Hydrogen loading in palladium wires was performed with the electrolysis in a 5 × 10-5M SrSO4 solution [11]. The palladium wire (cathode) was placed though the center of the platinum coil (anode). Prior to adding the electrolytic solution, the initial resistance was determined using a four-wire measurement with an LCR meter. The electric resistance of the wire was monitored during electrolysis and the ratio of the resistance of loaded state over that of the unloaded state was used to determine the hydrogen loading ratio (atomic ratio of H/Pd) in palladium based on well documented experimental data [12,13]. Extremely high loading has been achieved with this technique, approaching a factor of one hydrogen atom to each palladium atom. The current of the cell was approximately 4 mA and was applied for varying amounts of time to achieve different loading ratios. As an example, a typical loading time to the loading ratio of 0.85 took less than a half an hour, whereas loading to higher loading ratios took increasingly more time.
In the second part of the experiment, we applied an electrical pulse to the hydrogen loaded palladium wires in an effort to study the effects of hydrogen concentration on palladium nanoporosity. A relatively simple pulser was constructed from a small number of components which included a pressurized spark gap, charging resistor, high voltage supply (set to 20 kV), and a 2.5 nF, 40 kV capacitor. Figure 1 shows the schematic of the pulser and a typical current trace. A peak power of 13 Megawatts was delivered to the wire. However, the energy delivered to the wire was less than 0.7 J, which was inadequate to melt the 3.75 cm long and 50 μm thick unloaded palladium wire. This was verified with multiple wire pulsing experiments of the unloaded palladium wires. Moreover, all the unloaded palladium wires remained intact after being pulsed.
Discussion
The experiments with palladium hydride wires with varying hydrogen loading ratios were conducted with the same pulsing parameters for comparison. The experiments were conducted on single samples at each loading ratio. The debris from these experiments was analyzed using scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDAX), where only palladium was detected. In order to collect the debris from the pulsed wire, a plastic specimen bag was placed over the wire prior to the wire being pulsed. Figure 2 presents SEM images of palladium hydride wires or their debris after applying an electrical pulse. The palladium hydride wires have hydrogen loading ratios of 0.5, 0.6, and 0.8, respectively. At the hydrogen loading ratio of 0.5, there were no visible effects after pulsing; it was indistinguishable from a pulsed unloaded wire. The wire surfaces appear to be fairly smooth with no grain structures visible. However, at the loading ratio of 0.6, even though nanoporosity was not observed, we did observe the production of a large hole on the order of a few micrometers in the wire along with a noticeable change in the surface morphology. Grains of approximately ten micrometers can clearly be seen in the wire. One possibility is that the loose surface layer, resulting from oxidation in annealing and reduction in electrolysis was easily vaporized away in the wire pulsing experiment. Note that the pulsed wire becomes a two-phase body, consisting of a colder core that conducts lower current, and a hot surface region which conducts most of the current [14]. The overall hydrogen loading ratio of 0.6 appears to be the threshold of breaking palladium hydride wires by electrical pulsing. Of interest is that this threshold number closely agrees with the β phase boundary of palladium hydrides. As the hydrogen loading ratio was increased to 0.8, the wire started to break apart. Most of the wire disintegrated into tiny particles with only a few small pieces of the wire remaining partially intact. As shown in figure 2 (c), the surface of a piece of the wire which did not completely disintegrate was broken open and parts of the inside of the wire were ejected. The wire appears to fracture at the interface between grain boundaries, which would take less energy than a fracture of the crystal lattice structure. It was also observed that a small hole was produced at this loading ratio even within the grain, suggesting hydrogen atoms have a strong tendency to break out from the palladium hydride matrix during wire pulsing. At hydrogen loading ratios higher than 0.6, electrical pulsing of palladium hydride wires produced numerous debris (particles) with size dimensions on the order of 10 μm as observed under SEM. Further examination reveals that nanoporosity is present throughout the particles and debris. Figure 3 shows SEM images of debris of exploded palladium hydride wires at loading ratios of 0.8 and 0.96, respectively. At the loading ratio of 0.8, the debris retains a granular nature but surfaces of grains became very rough with pitting-like pores of approximately 100 nm which fall into the subcategory of nanoporous material refered to as macroporous as defined by the IUPAC [15]. More micrometer sized holes were also observed within the grains. At the loading ratio of 0.96, nanoporous palladium exhibits an extensive amount of pores with pore sizes down to 100 nm. (This extreme loading ratio is the only datum presented in which mercury was used to seal the wire.) The nanopores seem to be interconnected and appear to have a complex geometry. The granular nature is no longer present and the debris appears to be continuous although the size of it is much larger (~40 μm) than the typical grain size in the original wire. This probably results from strong shocks in the electrical pulsing of the wire, which had the highest experimentally achieved hydrogen loading ratio, approaching 1.
The observed experimental results indicate that the hydrogen loading ratio has significant effects on the formation of nanoporosity in palladium in electrical wire pulsing. On one hand, the process could have been altered due to the change of the electric conductivity of the palladium hydride wires for different loading ratios. On the other hand, large amounts of hydrogen loaded in palladium could result in significant changes of the mechanical properties of the palladium hydride wires. Computer simulations show that the tensile strength of palladium hydrides decreases with increasing hydrogen loading ratios [16]. In addition, nanometric bubbles can be formed in palladium, as evidenced by experimental observations of nanobubble production during tritium storage in palladium. Tritium, a radioactive isotope of hydrogen, decays to 3He, which is not soluble in palladium [17]. During an electrical pulsing, Joule heating induces a rapid increase of wire temperature and makes palladium hydrides thermodynamically unstable. In a very short time period, hydrogen atoms would be driven out from the palladium hydrides and result in the catastrophic and comprehensive rupture of the palladium, with the resulting formation of pervasive nanoporosity in the palladium debris. It is believed that shock waves would be generated during the wire pulsing process, especially when a large amount of hydrogen is loaded. A high hydrogen loading ratio not only makes for a strong shock and explosion but the palladium hydride is also easier to break because of decreasing mechanical strength.
Conclusion
In summary, we demonstrated that nanoporous palladium can be created in highly loaded palladium hydride wires by applying a low energy, fast electric pulse. Hydrogen loaded in palladium significantly affects the electrical wire explosion process. At a hydrogen loading ratio below 0.6, the palladium hydride wires remain intact, whereas at hydrogen loading ratios higher than 0.6, nanoporosity in palladium was observed. The higher the hydrogen loading ratio, the more extensive the nanoporosity produced. The novel method we used provides an alternative way to effectively produce nanoporous palladium.
Figures
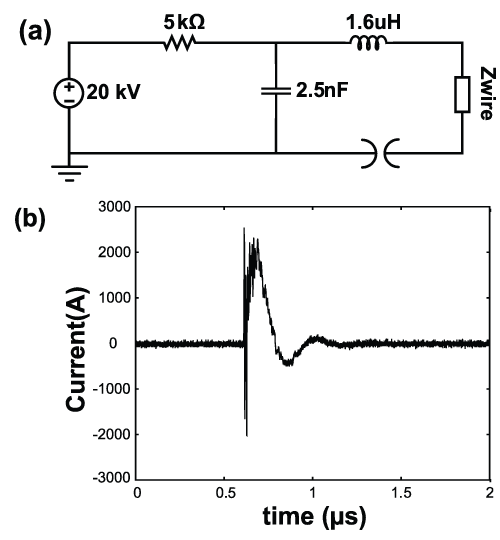
Figure 1: (a) Simple pulser circuit used during experiment. (b) Typical current delivered to the wire.
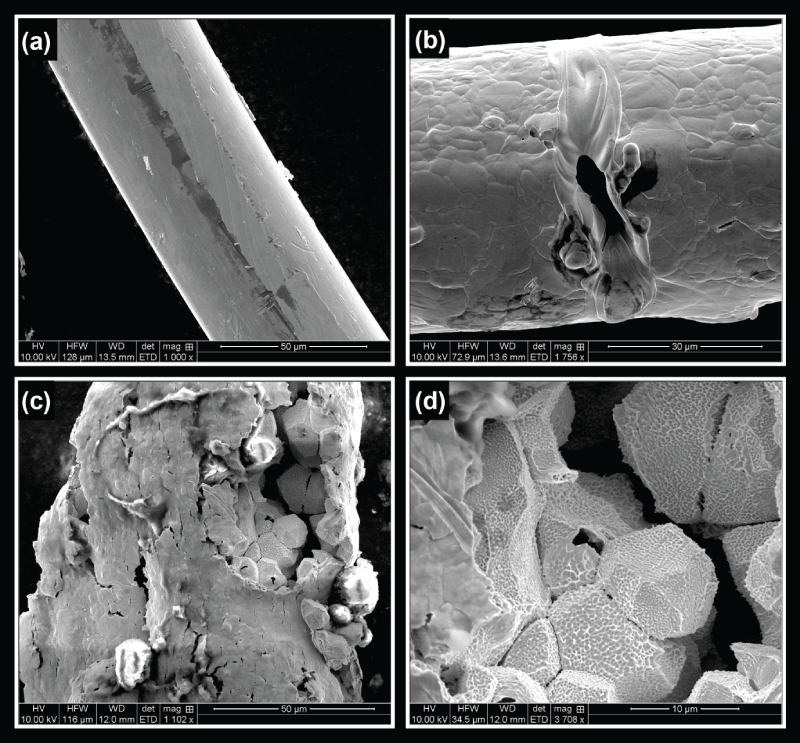
Figure 2: (a) 50% loaded wire after pulsing. (b) 60% loaded wire after pulsing. (c) 80% loaded wire after pulsing. (d) Higher magnification of 80% loaded wire after pulsing.
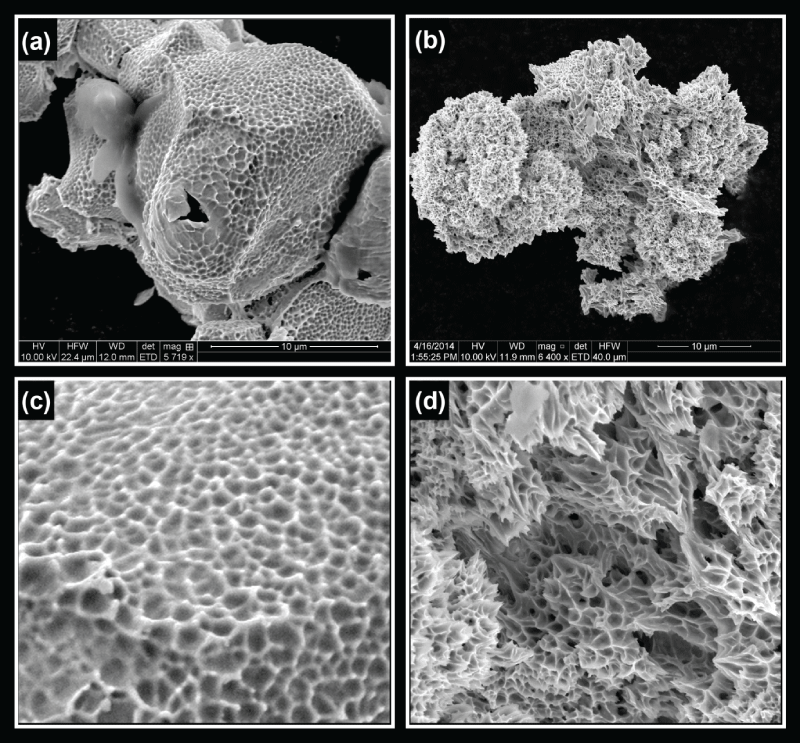
Figure 3: (a) 50% loaded wire after pulsing. (b) 60% loaded wire after pulsing. (c) 80% loaded wire after pulsing. (d) Higher magnification of 80% loaded wire after pulsing.
References
-
Hubert T, Boon-Brett L, Black G, Banach U (2011) Hydrogen sensors - A review. Sens Act B: Chemical 157: 329-352.
-
Hakamada M, Nakano H, Furukawa T, Takahashi M, Mabuchi M (2010) Hydrogen Storage Properties of Nanoporous Palladium Fabricated by Dealloying. J Phys Chem C114: 868-873.
-
Ding D, Chen Z (2007) A Pyrolytic, Carbon-Stabilized, Nanoporous Pd Film for Wide-Range H2 Sensing. Adv Mater19: 1996-1999.
-
Li W, Ma H, Huang L, Ding Y (2011) Well-defined nanoporous palladium for electrochemical reductive dechlorination. Phys Chem Chem Phys 13: 5565-5568.
-
Hakamada M, Mabuchi M (2009) Fabrication of nanoporous palladium by dealloying and its thermal coarsening. J Alloys Comp 479: 326-329.
-
Yu J, Ding Y, Xu C, Inoue A, Sakurai T, et al. (2008) Nanoporous Metals By Dealloying Multicomponent Metallic Glasses. Chem Mat 20: 4548-4550.
-
Robinson DB, Fares SJ, Ong MD, Arslan I, Langham ME, et al. (2009) Scalable synthesis of nanoporous palladium powders. Int J Hydrogen Energ 34: 5585-5591.
-
Gahl J, Baker J, Norgard P, Azizi O, El-Boher A, et al. (2014) Production of Nanoporous Palladium From a Deuterium Loaded Wire Driven By a Low Energy, Fast Pulser, IEEE IPMHVC 584-586.
-
Kotov YA (2003) Electric explosion of wires as a method for preparation of nanopowders. J Nanopart Res 5: 539-550.
-
Zheng G, Popov BN, White RE (1994) The Role of Thallium as a Hydrogen Entry Promoter on Cathodically Polarized HY-130 Steel. J Electrochem Soc 141: 1526-1532.
-
Tripodi P, McKubre MCH, Tanzella FL, Honnor PA, Di Gioacchino D, et al. (2000) Temperature coefficient of resistivity at compositions approaching PdH. phys letters A 276: 122-126.
-
Otterson DA, Smith RJ (1969) Absorption of hydrogen by palladium and electrical resistivity up to hydrogen-palladium atom ratios of 0.97. NASA Tech Note D-5441.
-
Baranowksi B, Wisniewski R (1969) The Electrical Resistance of Palladium and Palladium-Gold Alloy (50 wt% Au and Pd) in Gaseous Hydrogen up to 24000 at 25 C. Physica Stat Sol 35: 593-597.
-
Bennett FD (1968) High-temperature exploding wires, Progress in High Temp. Phys Chem 2: 3.
-
IUPAC. Compendium of Chemical Terminology (2nd edition). In: McNaught AD, Wilkinson A, The Gold Book. Blackwell Scientific Publications, Oxford (1997).
-
Zhou XW, Zimmerman JA, Wong BM, Hoyt JJ (2008) An embedded-atom method interatomic potential for Pd-H alloys. J Mat Res 23: 704-718.
-
Fabre A, Decamps B, Finot E, Penisson JM, Demoment J, et al. (2005) On the correlation between mechanical and TEM studies of the aging of palladium during tritium storage. J Nuc Mat 342: 101-107.
