
International Journal of Metallurgy and Metal Physics
(ISSN: 2631-5076)
Volume 5, Issue 1
Research Article
DOI: 10.35840/2631-5076/9247
Synthesis and Characterization of Cobalt Ferrite CoxFe3-xO4 Nanoparticles by Raman Spectroscopy and X-Ray Diffraction
A Flores Gil*, O Benavides, S Martínez Vargas, L De La Cruz May and C Patiño Carachure
Table of Content
Figures
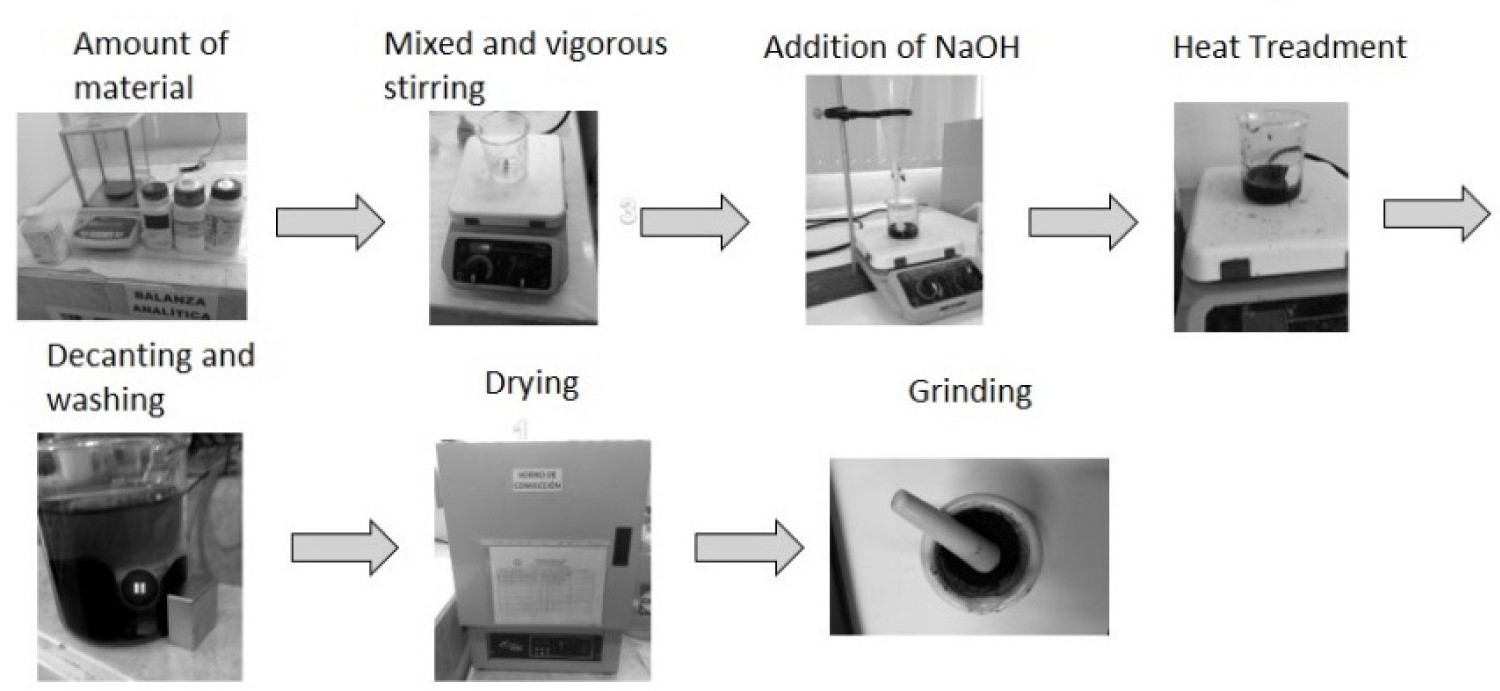
Figure 1: Cobalt ferrite nanoparticles synthesis.....
Cobalt ferrite nanoparticles synthesis process.

Figure 2: The upper Raman spectra shows four active modes.....
The upper Raman spectra shows four active modes of (A1g + Eg + 2T2g) expected for ferrite (M1), at bottom is clear that when Cobalt is included, A1g is spplited into two variant components, labelled as A1g(1) and A1g(2). T2g(2) have changes too. No clear evidence of T2g(1) and Eg are present for M2 and M3 spectra.
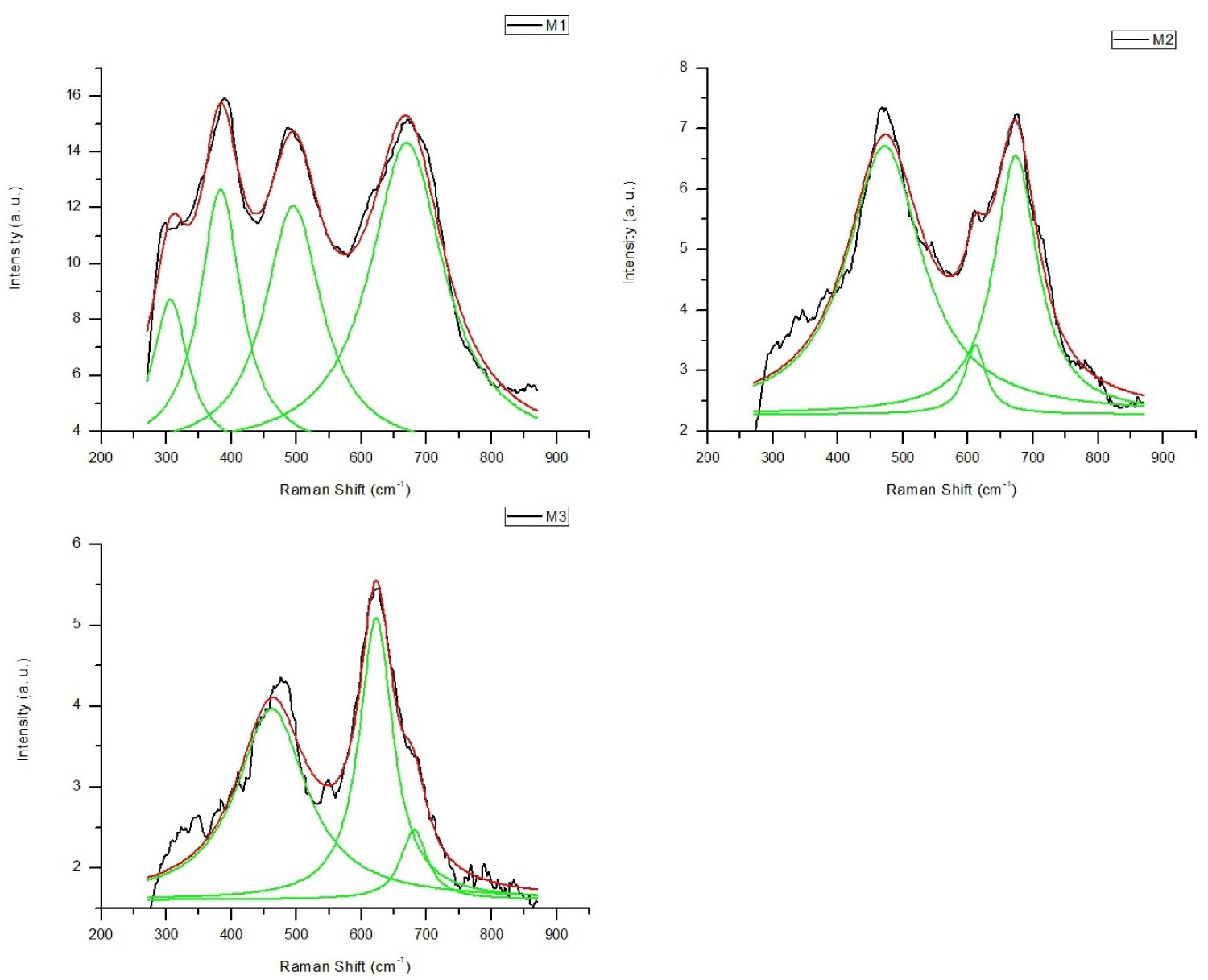
Figure 3: The best Lorenzian curves fitting the M1, M2.....
The best Lorenzian curves fitting the M1, M2 and M3 Raman spectrums. The major varibility on M2 and M3 are concentrated in the T2g and 2A1g active modes.
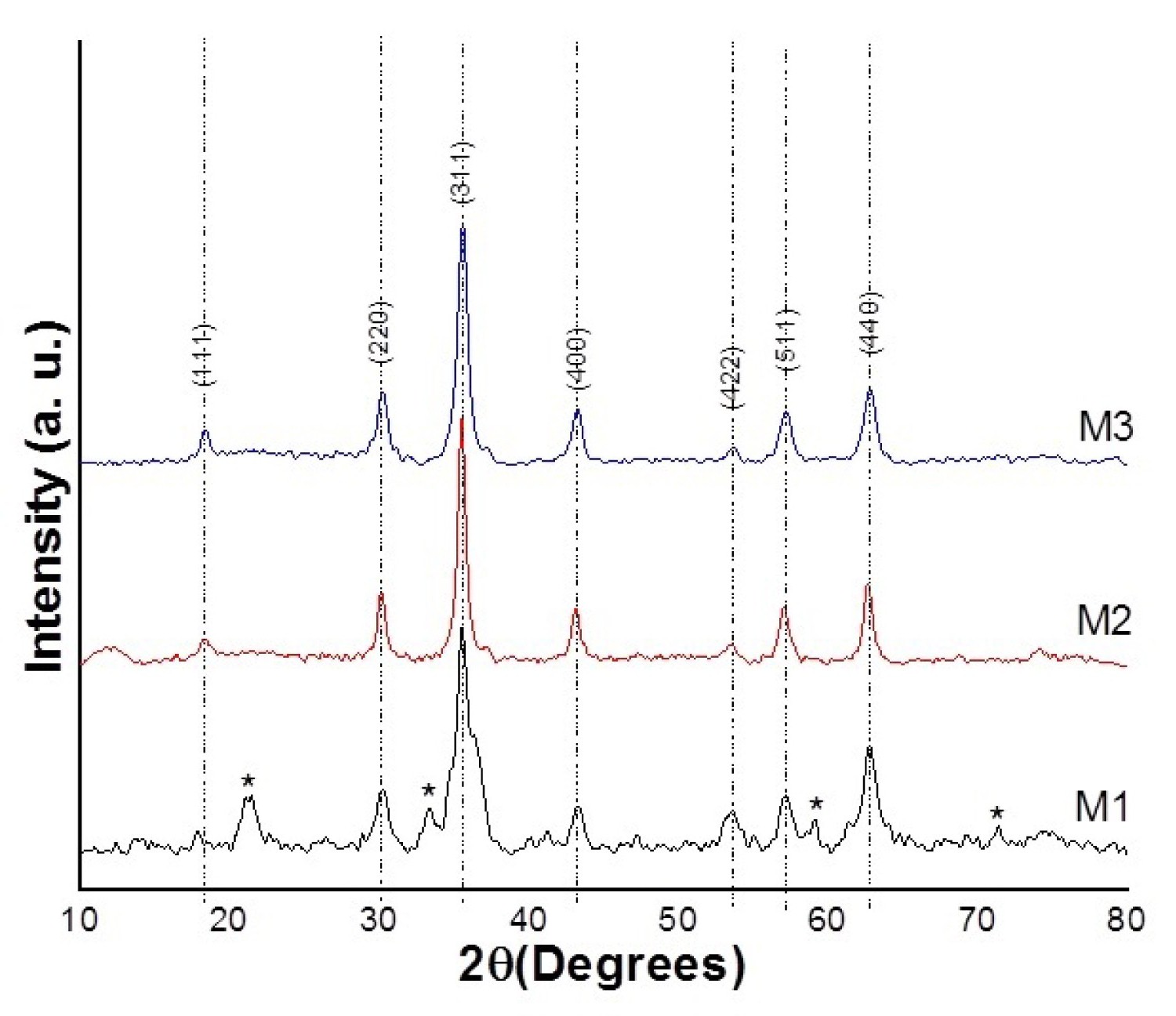
Figure 4: XRD Diffractograms of the grinding process.....
XRD Diffractograms of the grinding process.
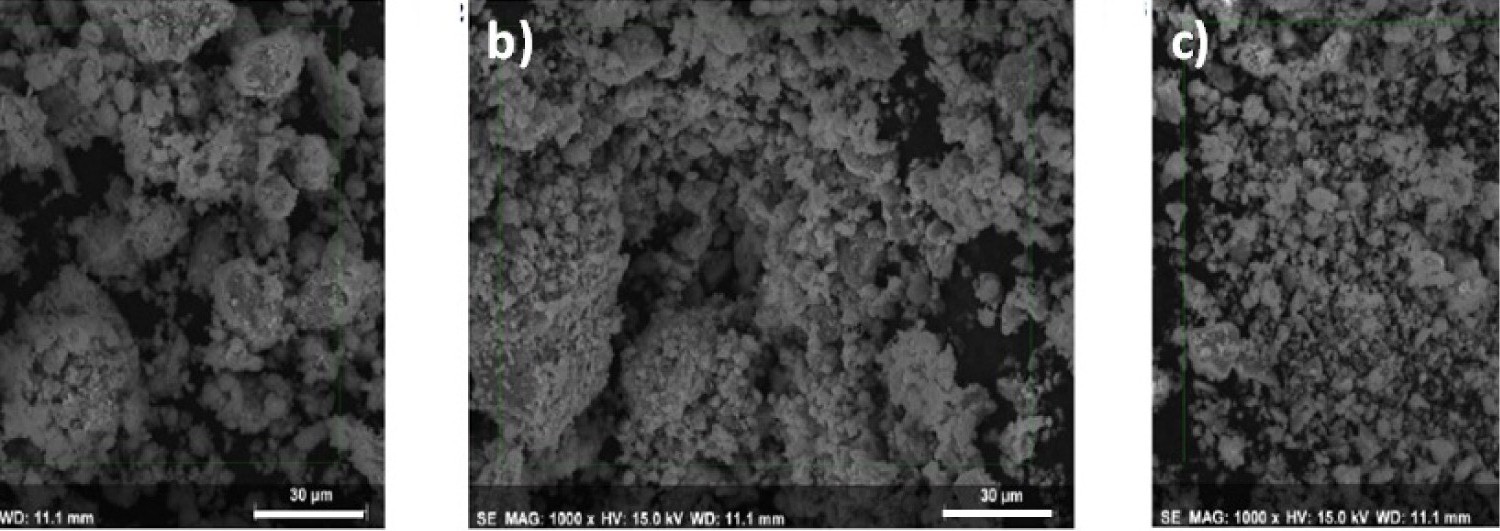
Figure 5: SEM micrographs, corresponding to....
SEM micrographs, corresponding to: a) M1; b) M2 and c) M3.
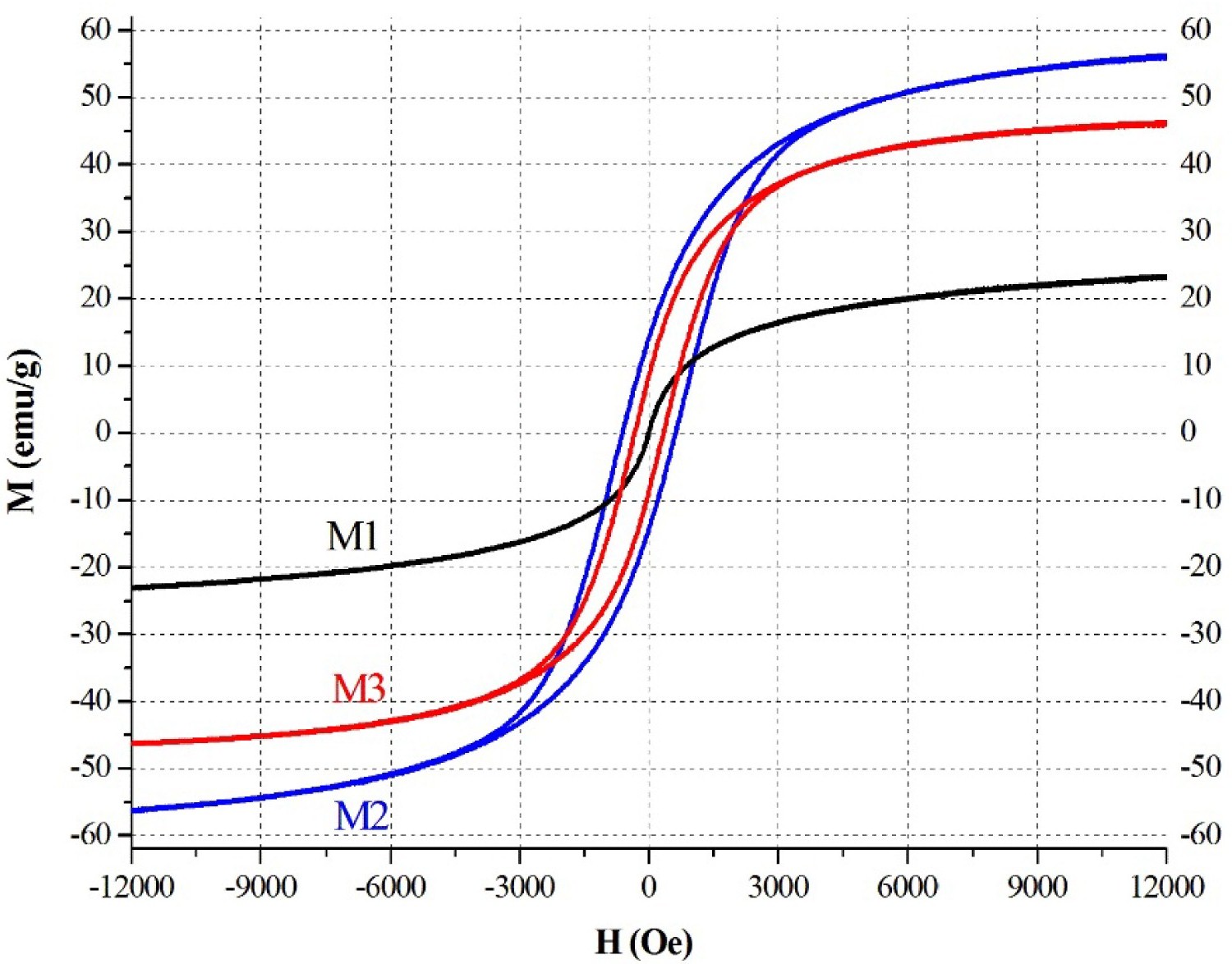
Figure 6: Hysteresis curve with specific magnetization at....
Hysteresis curve with specific magnetization at room temperature for M1, M2 and M3 NPs.
Tables
Table 1: Portions for the formation of CoxFe3-xO4.
Table 2: Raman modes for Cobalt Ferrite nanoparticles.
Table 3: Content of Fe, O and Co for the samples.
References
- Naik S, Salker A, Yusuf S, Meena S (2013) Influence of Co 2+ distribution and spin-orbit coupling on the resultant magnetic properties of spinel cobalt ferrite nanocrystals. Journal of Alloys and Compounds 566: 54-61.
- Tiwari S, Prakash R, Choudhary R, Phase D (2007) Oriented growth of Fe3O4 thin film on crystalline and amorphous substrates by pulsed laser deposition. Journal of Physics D: Applied Physics 40: 4943.
- Boles MA, Ling D, Hyeon T, Talapin DV (2016) The surface science of nanocrystals. Nature Materials 15: 141-153.
- Tung L, Kolesnichenko V, Caruntu D, Chou N, O'connor C, et al. (2003) Magnetic properties of ultrafine cobalt ferrite particles. Journal of Applied Physics 93: 7486-7488.
- Hyeon T, Chung Y, Park J, Lee SS, Kim YW, et al. (2002) Synthesis of highly crystalline and monodisperse cobalt ferrite nanocrystals. The Journal of Physical Chemistry B 106: 6831-6833.
- Ammar S, Helfen A, Jouini N, Fievet F, Rosenman I, et al. (2001) Magnetic properties of ultrafine cobalt ferrite particles synthesized by hydrolysis in a polyol medium. Journal of Materials Chemistry 11: 186-192.
- Tishin A, Buschow K (1999) Handbook of Magnetic Materials, Netherlands, 12.
- Byrne JD, Betancourt T, Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced Drug Delivery Reviews 60: 1615-1626.
- Gonzales-Weimuller M, Zeisberger M, Krishnan KM (2009) Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. Journal of Magnetism and Magnetic Materials 321: 1947-1950.
- Chen DX, Via G, Xu FJ, Navau C, Sanchez A, et al. (2011) Waiting time dependence of T-2 of protons in water suspensions of iron-oxide nanoparticles: Measurements and simulations. Journal of Applied Physics 110: 073917.
- Moumen N, Pileni M (1996) New syntheses of cobalt ferrite particles in the range 2-5 nm: Comparison of the magnetic properties of the nanosized particles in dispersed fluid or in powder form. Chemistry of Materials 8: 1128-1134.
- Chandramohan P, Srinivasan M, Velmurugan S, Narasimhan S (2011) Cation distribution and particle size effect on raman spectrum of CoFe2 O4. Journal of Solid State Chemistry 184: 89-96.
- Yue Zhang, Zhi Yang, Di Yin, Yong Liu, ChunLong Fei, et al. (2010) Composition and magnetic properties of cobalt ferrite nano-particles prepared by the co-precipitation method. Journal of Magnetism and Magnetic Materials 322: 3470-3475.
- Niéli Daffé, Fadi Choueikani, Sophie Neveu, Marie-Anne Arrio, Amélie Juhin, et al. (2018) Magnetic anisotropies and cationic distribution in CoFe2O4 nanoparticles prepared by co-precipitation route: Influence of particle size and stoichiometry. Journal of Magnetism and Magnetic Materials 460: 243-252.
- Indira TK, Lakshmi PK (2010) Magnetic nanoparticles-A review. Int J Pharm Sci Nanotechnol 3: 1035-1042.
- Yu T, Shen Z, Shi Y, Ding J (2002) Cation migration and magnetic ordering in spinel CoFe2O4 powder: Micro-raman scattering study. Journal of Physics: Condensed Matter 14: 613.
- Kreisel J, Lucazeau G, Vincent H (1998) Raman spectra and vibrational analysis of BaFe12O19 hexagonal ferrite. Journal of Solid State Chemistry 137: 127-137.
- Shebanova ON, Lazor P (2003) Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. Journal of Solid State Chemistry 174: 424-430.
- Jacintho GV, Brolo AG, Corio P, Suarez PA, Rubim JC (2009) Structural investigation of MFe2O4 (M= Fe, Co) magnetic fluids. The Journal of Physical Chemistry C 113: 7684-7691.
- Ramesh Kumar P, Young Hwa Jung, Kamala Bharathi K, Chek Hai Lim, Do Kyung Kim (2014) High capacity and low cost spinel Fe3O4 for the Na-ion battery negative electrode materials. Electrochimica Acta 146: 503-510.
- DLA de Faria, SV Silva, MT de Oliveira (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. Journal of Raman Spectroscopy 28: 873-878.
- Tozini D, Forti M, Gargano P, Alonso P, Rubiolo G (2015) Charge difference calculation in Fe/Fe3O4 interfaces from DFT results. Procedia Materials Science 9: 612-618.
- Yadav RS, Kuřitka I, Vilcakova J, Havlica J, Kalina L, et al. (2018) Sonochemical synthesis of Gd3+ doped CoFe2O4 spinel ferrite nanoparticles and its physical properties. Ultrasonics Sonochemistry 40: 773-783.
- Chand P, Vaish S, Kumar P (2017) Structural, optical and dielectric properties of transition metal (MFe2O4; M= Co, Ni and Zn) nanoferrites. Physica B: Condensed Matter 524: 53-63.
- Kumar A, Sharma P, Varshney D (2014) Structural, vibrational and dielectric study of Ni doped spinel Co ferrites: Co1- x Nix Fe2 O4 (x= 0.0, 0.5, 1.0). Ceramics International 40: 12855-12860.
- Yu Y, Mendoza-Garcia A, Bo Ning, Shouheng Sun (2013) Cobalt-substituted magnetite nanoparticles and their assembly into ferrimagnetic nanoparticle arrays. Advanced Materials 25: 3090-3094.
Author Details
A Flores Gil*, O Benavides, S Martínez Vargas, L De La Cruz May and C Patiño Carachure
Universidad Autónoma del Carmen, Cd. del Carmen, México
Corresponding author
A Flores Gil, Universidad Autónoma del Carmen, Cd. del Carmen, Campeche, 24150, México.
Accepted: March 07, 2020 | Published Online: March 09, 2020
Citation: Gil AF, Benavides O, Vargas SM, May LDLC, Carachure CP (2020) Synthesis and Characterization of Cobalt Ferrite CoxFe3-xO4 Nanoparticles by Raman Spectroscopy and X-Ray Diffraction. Int J Metall Met Phys 5:047.
Copyright: © 2020 Gil AF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In this paper, ferrite nanoparticles of formula CoxFe3-xO4 (where x = 0.0, 0.5 and 1) have been synthetized, through the chemical co-precipitation method, in a basic medium through the use of Fe (III), Fe (II) and Co (II) solutions. The powders were characterized by Raman spectroscopy and X-Ray Diffraction (XRD). The diffractograms indicated the Co (II) ions occupied the tetragonal region, modifying considerably the distribution of the rest of the ions present in the inverse spinel structure. Raman spectra shows variations in the intensity of the characterized bands located at 460, 624 and 672 cm-1 for each structure.
Keywords
Nanoparticles, Cobalt ferrites, Raman spectroscopy, Ion dispersion and displacement
Introduction
As nanoscience and nanotechnology progress, new sort of magnetic nanocrystals are being created, among these paramagnetic nanomaterials, ferrite nanoparticles stand out, due to their unique fascinating properties, such as magnetic, electronic and magneto-optic [1], which exhibit a good combination of high electric resistance and ferromagnetic behavior [2]. The novel physicochemical and electromagnetic properties, observed in the ferrite nanoparticles, are caused by the changes in the composition and size of the particles. These peculiar properties have led researchers to synthesize these materials [3]. The facility with which these materials can be synthesized and the stability of the same under diverse conditions, have caused great interest in many industrial areas [4,5]. The study of magnetic nanocrystals has been fundamental for the comprehension of nanomagnetism, so it can be applied in different areas such as; medicine and biomedical science [6,7]. Can be a useful tool for the development of therapeutic agents, carcinogenic diagnostics [8], hyperthermia with magnetic fluids, contrast agents for magnetic resonance images [9,10] and antimicrobial agents [11].
The nanocrystal family belonging to the spinel and inverse spinel ferrites is represented by the molecular formula AB2O4 [1]. The magnetic properties of these oxides are mainly governed by the distribution and interaction of the cations in their both tetrahedral and octahedral networks [1], for example; the migration of the divalent ions such as Co, Ni, Mg and Zn form the CoFe2O4, MgFe2O4 and MnFe2O4 nanoferrites [12].
The cobalt ferrite nanoparticles have been synthesized by the co-precipitation method through changing the adding sequences of the reactants, the concentrations of NaOH solutions, as well as the temperatures in the subsequent annealing treatment. The mechanism for the composition and size dependence of magnetic properties was studied in detail [13].
Magnetic CoFe2O4 nanoparticles with different sizes were obtained from the versatile coprecipitation process. Studying the temperature treatments or with the addition of tartrate ions, Fe nitrates or Co nitrates, nanoparticle sizes were tuned between 7 nm and 21 nm [14].
In this work, by using the co-precipitation method, magnetic nanoparticles of magnetite and cobalt ferrites were prepared. Co (II) accomplished gradual replacement of the metallic ions Fe (II), forming the general molecular system of nanoferrites CoxFe3-xO4, where the stoichiometric relation of Co2+ was used (0.0, 0.5, and 1.0) in the ferrite's crystalline network. Therefore, the influence of Co (II) ions in the inverse spinel structure is demonstrated through Raman spectroscopy and X-Ray Diffraction techniques.
Description of the Method
Synthesis of cobalt ferrite nanoparticles
Currently there is a vast diversity of methods that can be used for the preparation of magnetic nanoparticles with sizes that of 10 nm approximately [15,16], the simplest and viable method is chemical co-precipitation. This method is based on an aqueous solution mixture of suspended salts in an alkaline medium (see Figure 1).
8 mmol of Fe (III) were dissolved in 5 ml of deionized water, this solution was then mixed with the quantities indicated in Table 1 for Fe (II) and Co (II), this was later dissolved in 5 ml of water slightly acidulated (pH = 4). For M1 4.0 mmol of FeSO4∙7H2O was dissolved in 2.5 mL of deionized water acidified with 0.5 ml of HCl 1.0 N. A solution containing 8.0 mmol Fe(NO3)3∙9H2O dissolved in 2.5 mL of deionized water acidified with 0.5 mL of HCl 1.0 N was added under vigorous stirring. The M2 were synthesized using the described methodology, except that the 4.0 mmol FeSO4∙7H2O was replaced by 2.0 mmol of FeSO4∙7H2O and 2.0 mmol of Co(NO3)∙6H2O. Accordingly, for M3 4.0 mmol of FeSO4∙7H2O was replaced by 4.0 mmol of Co(NO3)∙6H2O. The solutions were mixed and vigorously agitated for 10 minutes at a stable temperature (between 20 and 25 ℃). Later, drop by drop, 100 ml of NaOH at 3.0 M were slowly added as a precipitating agent. During the precipitation, the solution's colour changed to black, distinctive of this material. This indicates the formation of a ferrofluid. The next step is to raise the temperature to 90 ℃ and maintain it for an hour while it is being vigorously agitated. Later, the solution has to be cooled down at room temperature and washed three times with deionized water. Once the sample is washed, the next procedure is to let it dry for 24 hours at 90 ℃, and then the sample is pulverized in a mortar so it can finally be characterized by Raman spectroscopy and XRD.
Cobalt ferrites nanoparticles characterization
The characterization of the magnetic nanoparticles is important, for this reason, the Raman spectroscopy and the X-Ray Diffraction were used to obtain information at a molecular level. Characterizations of the ferrite nanoparticles morphology and elemental composition were analysed on a SEM HITACHI S-3400N fitted with an electron dispersive X-ray (10 Kv and 30 pA; image magnification 1500X and the work distance of 10.5 mm). In addition, the atomic ratio of cobalt and iron was determined by a Perkin Elmer Optima 7000 DV ICP-OES Spectrometer. Where the Raman spectroscopy, is a non-destructive method that does not require sophisticated preparation to analyse samples, for the case of the magnetic nanoparticles. In addition, it is a powerful and sensible tool for the analysis of thin films and powders. This technique is useful to comprehend the microstructure in nanomaterials, structure transition, network distortion, charge and reticle of lattice couplings, the distribution of local cation and the magnetic arrangement [1,12].
In this research, a near infrared laser with a wavelength of 785 nm was used to produce the laser beam, and, to obtain information from the samples, an Ocean Optics spectrogram model QE65000 was adjusted to an interval of 0 to 2100 cm-1, with a spectral resolution of 3-4 cm-1. The integration time and the fixed potency of the laser were 125 seconds and 35 mW, respectively.
Regarding the X-Ray Diffraction instrument, a Rigaku UIV of 40 kV and 30 mA, with a wavelength corresponding to the Cu Kα radiation, which is λ = 1.5406 Å, was used to obtain the diffractograms, which were mapped from 5 to 80° in 2θ with a step of 0.02°.
Results and Discussion
The Ferrite Raman spectrum (M1) possesses a group of factors (phonon modes belonging to the inverse spinel structure) such as; A1g(R), Eg (R), T1g, 3T2g (R), 2A2u, 2Eu, 4T1u (IR) and 2T2u [14]. When analysing the group of factors, only a certain range of the spectrum can be observed with the instrument, which consists of four active Raman modes 1A1g+1Eg+2T2g [17,18]. Which correspond to the bands of 306, 393, 495, y 669 cm-1, respectively [19].
In Figure 2, The upper Raman spectra shows four active modes of (A1g + Eg + 2T2g) expected for ferrite Fe3O4 (M1) [20], at bottom is clear that when Co2+ substitutes the Fe2+ ions A1g is splitted into two variant components, labelled as A1g(1) and A1g(2), as expected for CoFe3O4 [21], T2g(2) have changes too. Although, there are no clear evidence about T2g(1) and Eg in M2 and M3 spectra.
Figure 2, shows also a remarkable difference between M2 and M3 respect to M1, Raman bands of M2 and M3 are less intense than Raman bans of M1, but with the particularity that A1g is splitted into to modes. For M1 which only contains half of the amount of Co (II) in relation to Fe (II), A1g(2) is shifted to lower energy bands, around 624 cm-1, and A1g(1) is shifted to higher energy bands, around 672 cm-1. In this case, A1g(2) intensity < A1g(1) intensity. Fort the case of M3 which contains the 100% of Co, also are appreciable the A1g(2) and A1g(1) modes, but A1g(2) intensity > A1g(1) intensity. Thus, the changes in the intensity of vibrational modes in this particular case, could indicate the redistribution of cations in the tetrahedral spaces (T) of Fe3+ to the octahedral spaces (O) of Co2+, which alters the symmetry of the crystalline structure [22].
Figure 3, shows the best Lorentzian fits on M1, M2, and M3 spectrums. Inspection of Lorentzian curves on T2g(2), A1g(1) and A1g(2) depict important variations, with little shift center, and changes in peak intensities as Cobalt increased. Table 2, shows the peak center variations of T2g(2), A1g(1) and A1g(2), and the relative intensities of the A1g (1) and A1g (2) modes.
A1g (1) and A1g (2) demonstrate the stretching vibrations of the Fe-O and Co-O e bonds in tetrahedral sites. The cation redistribution can be noticed by comparing the relative intensities of the A1g (1) and A1g (2) modes. From Figure 3 and Table 2, M3 sample has higher value in the relative intensity that agrees with the high percentage of Co(II). Consequently, A1g(2) is associated to the Co-O bonds [23]. While M2 sample has a lower value in the relative intensity, indicating that A1g(1) mode depends on the interaction of Fe-O bonds [24].
T2g(2) mode demonstrate the variations of the spinel structure, from Figure 3 and Table 2 is noticeable the shift to lower energy bands as Co increases. It is associated with the decreased of particle size in the sample [25].
On the other hand, in Figure 4, the diffraction patterns of the ferrite nanoparticles X-Rays can be observed. When analysing and indexing the diffractograms, it was observed that these matched with the magnetite standard by checking with the DRX 99-203-7367 card, this one also indicates that they possess an inverse spinel structure. The asterisk peaks of M1 diffractogram in Figure corresponding to other iron oxides phases (as goethite and maghemite). Similarly, to the analysis of the Raman spectra, the analysis for the diffractograms starts with the sample M1, which serves as a starting point. When comparing the patterns for M1, M2 and M3, a slight displacement in the 2θ angle was observed, due to the progressive substitution of Fe (II) over Co (II). To gather more information from the diffractograms, the greater intensity profiles were measured to obtain an approximate size of the ferrite nanoparticle, by making use of Debye Scherrer's equation (D = 0.9 λ/β cos θ), where D is the crystal size, λ is the wavelength of X-ray, θ is the Braggs angle in radians, and β is the full width at half maximum of the peak in radians. The results for the measurements of the next samples; Fe3O4, Co0.5Fe2.5O4 and CoFe2O4 (M1, M2 and M3 respectively), were; 13.02, 17.38, 13.29 nm, respectively.
Figure 5 shows a typical bright field SEM image of the sample, where numerous faceted particles with size of ~50 μm and agglomerates of particles smaller than 5 μm are distinguished. Dynamic light scattering (DLS), using a Nanotrac 252 Particle Size Analyzer, showed that the particle size is beginning with 2.375 nm for M1, 2.385 nm for M2 and 2.556 nm for M3.
The presence of cobalt in ferrite particles composition from SEM was corroborated by energy-dispersive X-ray spectroscopy (EDS) analysis, which displayed particles content to be 37.17% Fe and 62.83% O for M1, 13.31% Co, 31.59% Fe and 55.10% O for M2, and 20.82% Co, 23.60% Fe, and 55.58% O for M3. The elemental composition of cobalt in ferrite nanoparticles was also corroborated by ICP-OES, where nanoparticles were previously digested in concentrated hydrochloric acid. ICP-OES showed M2 and M3 nanoparticles to content 12.87% and 19.96% atomic Co, % for M2 and M3 respectively, in accordance to EDS, that's it, Co and Fe having a near ¼ and ½ molar ratio for M2 and M3 respectively, see Table 3. These results corresponded to with cobalt ferrites nanoparticles CoxFe3-xO4 (where x = 0.6, 1, 1.8) obtained by a very simple non-aqueous one-pot process [25].
Regarding magnetic properties, Figure 1b displays the magnetic hysteresis loops of the ferrite NPs. The data such as saturation magnetization (Ms), the coercive field (Hc), and the remanence (Mr) of the NPs can be extracted from the loops. The Ms of 23.7, 56.4, and 43.8 emu/g was determined for M1, M2, and M3, respectively. The Hc of 2.6, 618, and 251 Oe was determined for M1, M2, and M3, respectively. The Mr of 0.05, 7.5 and 14.0 emu/g was determined for M1, M2, and M3, respectively. The Hc and Mr values near zero for the M1 NPs indicate their superparamagnetic nature at room temperature. For the cobalt ferrites, the hysteresis loops reveal their ferrimagnetic nature, where the M2 NPs are magnetically harder than the M3 NPs. Variations of coercive fields of the cobalt ferrites NPs, where a maximum was found, and it was explained that M2 is magnetically harder than M3 and, in agreement with our results [26] Figure 6.
Conclusions
In this research three ferrite compounds (M1, M2 and M3) were synthetized, differing from one another in their Co(II) and Fe(II) percentages, through co-precipitation methodology. These compounds were characterized through Raman Spectroscopy and X-Ray Diffraction and their magnetic properties were determined thorough magnetometry. The characterization of the compounds helped to observe and determine valuable information from the phenomenon that is caused by the substitution of Fe2+ ions from their octahedral spaces by the Co2+. It was determined that the substitution of Fe2+ ions by Co2+ ions cause fluctuations in the intensity of the vibrational modes, intensity seems to decrease for T2g(2) as the percentage of Co(II) increases and Fe(II) decreases, this is due to the band 460 cm-1 being dependent on the interactions with the Fe-O bonds. Changes in intensity and peak center shift on the A1g(2) and A1g(1) modes demonstrating the stretching vibrations of the Fe-O and the Co-O bonds in the tetrahedral sites. It can be associated with the changes in the particle size, that agrees with the finding out analysis of the diffractograms by DLS.

